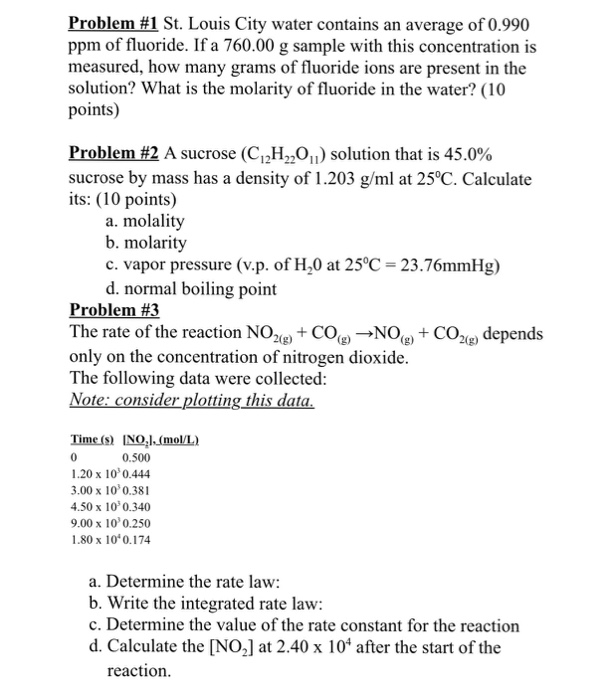
Students also viewed these Physical Chemistry questions. When 1410g of an unknown non-electrolyte compound was dissolved in 2820g of ethanol the solution was found to have a boiling point of 78833oC.

Lactose Structure C12h22o11 Over 100 Million Chemical Compounds Mol Instincts
Rank the boiling points of the following compounds from lowest to highest.
C12h22o11 boiling point. What is the boiling point of a 055 molal m sucrose C12H22O11 solution. What is the molar mass of the. Calculate the freezing point of a solution containing 0600 kg of CHCl3 and 420 g of eucalyptol C10H18O a fragrant substance found in the leaves of eucalyptus tree.
The term is used in the Solution properties 1. 0512C - Kb of H2O 2. Answers The symbol Na comes from the Latin word natrium.
533E-017 Modified Grain. Show all operations and write the result in units. It has the molecular formula C 12 H 22 O 11.
A solution contains 81 g of sucrose C12H22O11 a nonelectrolyte dissolved in 274 g of water. C12H22O11 sucrose O2 NaF asked Aug 27 2019 in Chemistry by toya4me A. Cellulose is an odorless white powdery fibers.
25542 Mean or Weighted MP VPmm Hg25 deg C. Further explanation Solution properties are the properties of a solution that dont depend on the type of solute but only on the concentration of the solute. Molal m that is the number of moles of solute in 1 kg of solvent 2.
What would be the boiling point of a solution in which 870 g of sucrose C12H22O11 were dissolved in 255 g of water. Pure water solution of C12H22O11 m001 in water solution of NaCl m001 in water solution of CaCl2 m001 in water choose the one with the a highest freezing point b lowest freezing point. Calculate the boiling point of a solution containing 12g glucose C6H12O6 dissolved in 200g of water H2O.
Answer in units of degrees Celsius. Log Octanol-Water Partition Coef SRC. F for CHCl3 is 468 0cmolal and normal freezing point is -635C.
Use the formula of the salt to obtain i. Journal Publishers via MeSH. What is the boiling-point elevation.
The boiling point. Both solutions have a higher boiling point than pure water. Calculate the molality of the solution Moles C2H22O11 in 960 g 960 g 3423 gmol 0280 mol dissolved in 0383 kg water molality 0280 mol 0383 kg water 0732 mol kg OR 0732 m The boiling point constant for water is 052 Cm.
59167 Adapted Stein Brown method Melting Pt deg C. C12H22O11 is a non-volatile non-dissociating solute. Sucrose is produced naturally in plants from which table sugar is refined.
Log Kow KOWWIN v167 estimate -512 Boiling Pt Melting Pt Vapor Pressure Estimations MPBPWIN v142. By signing up youll get thousands of step-by-step. Predicted data is generated using the US Environmental Protection Agencys EPISuite.
What is the boiling point of a solution of 1150 g of sucrose C12H22O11 in 3500 g of watera Outline the steps necessary to answer the questionb Answer the question. What is the boiling point of a 695 m solution of C12H22O11 in H2O. Calculate the normal freezing point of a 07439 mol aqueous solution of C12H22O11 that has a density of 135 gml.
Which flask will have a lower freezing point. O2 C12H22O11 sucrose NaF. Suppose you have two flasks one with pure water and one with a sucrose solution.
The boiling point of 1 M NaCl solution is higher than that. Predict the major product of the following radical monohalogenation reaction. The freezing point of pure paradichlorobenzene is 530C and the freezing point.
The reaction for the metabolism of sucrose C12H22O11 is the same as for its combustion in oxygen to yield CO2g and H2Ol. The biopolymer composing the cell wall of vegetable tissues. It is a disaccharide a molecule composed of two monosaccharides.
What is the boiling point of a solution made by adding 669 g of magnesium chloride to 2433 g of water. Calculate the boiling point and freezing point of a 250 mass percent solution of ethylene glycol in water. Sucrose is made up of one molecule of glucose and one molecule of fructose joined together.
Boiling Pt deg C. Prepared by treating cotton with an organic solvent to de-wax it and removing pectic acids by. Sucrose is a glycosyl glycoside formed by glucose and fructose units joined by an acetal oxygen bridge from hemiacetal of glucose to the hemiketal of the fructoseIt has a role as an osmolyte a sweetening agent a human metabolite an algal metabolite a Saccharomyces cerevisiae metabolite an Escherichia coli metabolite and a mouse metabolite.
The boiling point of ethanol CH3CH2OH is 78500oC at 1atm. What is the molality of C12H22O11 with a boiling point of 100 degrees Celcius. The standard heat of formation of sucrose is.
Kb 052oCm for water Group of answer choices A 100 oC B 10035 oC C 10052 oC D 10091 oC E 10041 oC. What is the boiling point of a 695 m solution of C12H22O11 in H2O.

What Is The Boiling Point Of An Aqueous Solution Of Chegg Com

Sucrose Properties Production And Application

Equation For C12h22o11 H2o Sucrose Water Youtube

Name Colligative Properties Molality M Moles Of Solute Kilogram
A Sucrose C12h22o11 Solution That Is 45 0 Sucrose Chegg Com

Equation For C12h22o11 H2o Sucrose Water Youtube
B 1 6 Galactobiose C12h22o11 Chemspider

25 A Solution Is Prepared By Dissolving Of 4 9g Chegg Com

Sucrose Structure C12h22o11 Over 100 Million Chemical Compounds Mol Instincts

Sugar C12h22o11 By Connor Scott
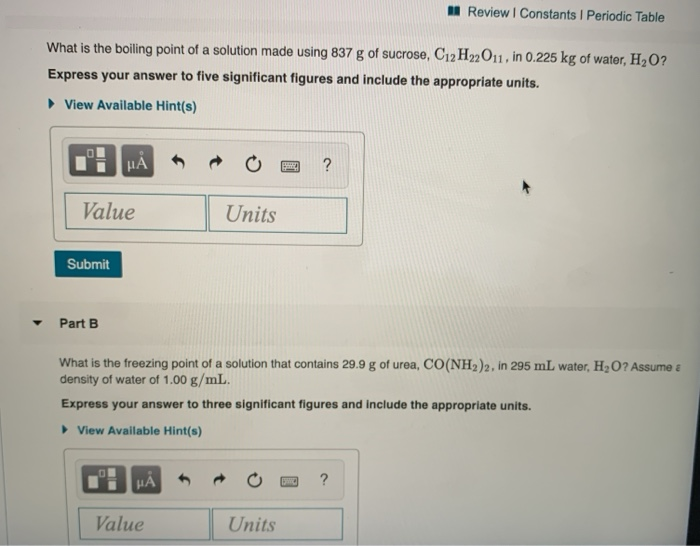
What Is The Boiling Point Of A Solution Made Using Chegg Com